Bonding Suggestions for the Piezoelectric Motor System
James Friend
210A ME Annex Building
University of Missouri-Rolla, Rolla, MO 65401
Dan Stutts
204A ME Building
University of Missouri-Rolla
May 1995
1. Abstract
Recently, the failure of bonds between the piezoelectric layers and the stator
have been identified as one of the primary failure modes of the piezoelectric
motor system. Attempts to alleviate this problem have been met with
frustration. This short paper is being provided as a guide for recommended
procedures and materials to bond the components of the motor together. It
condenses some information located in Joining of Advanced Materials by
Robert Messler,Jr.[1]
2. Bonding Improvement Suggestions: An Executive Summary
Improvement of the bond according to Messler can be accomplished by using some
of the following suggestions:
1. Prime the surfaces of the layers to be bonded, i.e., cover the
surfaces with a dilute solution of the adhesive mixed with an organic solvent
to obtain a dried film thickness between 0.0015 to 0.005 mm (0.00006 to 0.002
in) and cure separated before applying the adhesive to bond the layers
together.
2. Consider another adhesive. Epoxies are best, but an alloy such as
epoxy-phenolic or epoxy-polysulfone may offer improved peeling resistance.
3. Dilute the adhesive until it has a lower surface tension than either of the
bonding surfaces. The surface tensions can be compared by using a wetting
test, i.e., by wetting the surface with the adhesive and measuring the
"beading" angle. A low beading angle indicates good wetting and an appropriate
adhesive. See Figure 2.1.

Figure 2.1 Beading/adhesion relationship
3. Bonding Adhesives for the Motor System
3.1 Theories of Bonding
3.1.1 Summary
Many theories have been developed to explain the process of bonding in
adhesive structures. Individually, each of these theories is inadequate to
describe the complete process of bonding in most situations. However, each
theory contributes an understanding of the overall process of bonding and
therefore are important. Figure 3.1 illustrates the five predominant
mechanisms of adhesion.

Figure 3.1 Dominant mechanisms of bonding (from [1])
3.1.2 Mechanical Bonding
According to the mechanical bonding theory, an adhesive must fill the valleys
and crevices (the asperities) of each adherend (body to be bonded) and
displace trapped air to work well. Adhesion is the mechanical interlocking of
the adhesive and the adherend together, and the overall strength of the bond is
dependent upon the quality of this interlocking interface. To this end,
chemical or physical abrading is highly recommended for optimum bonding.
Abrading the adherend (1) enhances the mechanical interlocking, (2) creates a
clean corrosion-free surface, (3) forms a chemically reactive surface, and (4)
increases bonding surface area. Diffusion bonding is a form of mechanical
interlocking which occurs at the molecular level in polymers.
3.1.3 Adsorption Mechanism Theory
The adsorption mechanism theory suggests that bonding is the process of
intermolecular attraction (van der Waals bonding or permanent dipole, for
example) between the adhesive and the adherend at the interface. An important
factor in the strength of the bond according to this theory is the wetting of
the adherend by the adhesive. Wetting is the process in which a liquid
spreads onto a solid surface and is controlled by the surface energy of the
liquid-solid interface versus the liquid-vapor and the solid-vapor interfaces.
In a practical sense, to wet a solid surface, the adhesive should have a
lower surface tension than the adherend.
3.1.4 Electrostatic Theory
Electrostatic forces may also be a factor in the bonding of an adhesive to an
adherent. These forces arise from the creation of an electrical double layer
of separated charges at the interface and are believed to be a factor in the
resistance to separation of the adhesive and the adherend. Adhesives and
adherends that contain polar molecules or permanent dipoles are most likely to
form electrostatic bonding according to this theory.
3.1.5 Weak-Boundary Layer Theory
This theory has been developed to explain the curious behavior of the failure
of bonded materials. Upon failure, many adhesive bonds break not at the
adhesion interface, but slightly within the adherend or the adhesive, adjacent
to the interface. This suggests that a boundary layer of weak material is
formed around the interface between the two media. Impurities in the bond and
adverse chemical reactions are common causes of weak boundary layers.
3.2 Mechanisms of Failure in Bonded Joints
3.2.1 Mechanisms of Failure
The two predominant mechanisms of failure in adhesively bonded joints are
adhesive failure or cohesive failure. Adhesive failure is the interfacial
failure between the adhesive and one of the adherends. It indicates a
weak-boundary layer often from improper surface preparation or adhesive choice.
Cohesive failure is the internal failure of either the adhesive or, rarely, one
of the adherends. The difference is illustrated in Figure 3.2.
Ideally, the bond will fail within one of the adherends or the adhesive. This
indicates that the maximum strength of the bonded materials is less than the
strength of the adhesive strength between them. Usually, the failure of joints
is neither completely cohesive nor completely adhesive. Measurement of the
success of a particular joint is based on the relative percentage of cohesive
failure to adhesive failure.
3.2.2 Causes of Premature Failure
The precise cause of premature failures in adhesively bonded joints is
difficult to determine. The variety of causes include adverse stresses
(peeling, for example), rate of application of stresses, fatigue, temperature,
humidity, and solvents. Tests are required to evaluate the cause of adhesive
failures for most bonded systems.

Figure 3.2 Adhesive and cohesive failure of bonded joints
3.3 Key Requirements for Quality Bonding
All foreign materials, such as dirt, grease, cutting coolants and lubricants,
water or moisture, and weak surface scales (e.g., oxides, sulfides), must be
removed. Thorough cleaning with various physical or chemical processes removes
these contaminants and conditions the surface for bonding. The process of
cleaning the surface usually involves several of the following three steps:
(1) solvent cleaning; (2) intermediate chemical and/or mechanical cleaning;
and (3) chemical treatment. Priming may also be necessary.
Solvent cleaning is the process of removing soil from the surfaces from the
adherend using an organic solvent without physically or chemically altering it.
The following four processes employ solvent cleaning in an increasing order of
severity:
1. Vapor degreasing. Hot solvent in vapor form condenses on the object to be
treated and flows away debris.
2. Solvent wiping, immersion, or spraying.
3. Ultrasonic vapor degreasing. Ultrasonic excitation of thin layer of
solvent on object. Cavitation of bubbles on surface of object provides
scrubbing action.
4. Ultrasonic cleaning in solvent. Ultrasonic excitation with subsequent
solvent rinse of object.
Intermediate treatment of the surface often includes grit blasting, wire
brushing, sanding, abrasive scrubbing, scrapping or filing, and alkaline or
detergent cleaning. These cleaning methods may be aggressive enough to remove
some of the parent material.
Chemical treatment commonly includes acid or alkaline etching of the adherend
surface. Etching the adherend removes stubborn oxides and roughens the surface
on a microscopic scale. Solvent cleaning should always be performed before
chemical treatment.
Occasionally, priming the surface may be necessary. Priming is the
process of applying a dilute solution of the adhesive mixed with an organic
solvent on the adherend to a dried film thickness between 0.0015 to 0.005 mm
(0.00006 to 0.002 in). Priming protects the surface from oxidation, improves
wetting, helps prevent adhesive peeling, and serves as a barrier layer to
prevent undesirable reactions between the adhesive and the adherend.
3.4 Adhesive Selection
Most adhesives in use are actually a combination of components each designed
for different functions in the bonded joint. An adhesive can contain any
number of the following components: (1) the adhesive base or binder; (2) a
hardener (for thermosetting types); (3) accelerators, inhibitors, or
retardants; (4) diluents; (5) solvents; (6) fillers; and (7) carriers or
reinforcements.
The adhesive base is generally the primary component of any adhesive and is
the constituent from which the name of the adhesive is derived. A hardener is
a substance added to the adhesive that causes a chemical reaction which cures
the adhesive. Accelerators, inhibitors, and retardants control the adhesive's
rate of curing. Diluents are liquid components that are added to the adhesive
to reduce the concentration of the adhesive base. They usually reduce the
viscosity of the adhesive. Solvents are closely related in that they thin the
adhesive to make it more spreadable. The difference is that solvents
disperse the adhesive base. Fillers are included in the adhesive if the
adhesive properties are insufficient alone. Often, fillers are used for
reducing cost, improving structural strength, encouraging conductivity, and
other similar applications. Carriers or reinforcements are used to support the
adhesive during application or storage.
Adhesives can be classified into a number of different categories, but the
most convenient system is categorization by chemical composition. Figure 3.3
shows a summary of major structural adhesives categorized by the Society of
Mechanical Engineers. Figure 3.4 and 3.5 tabulate the appropriate adhesives to
use for particular adherends.

Figure 3.3 Categorization of Structural Adhesives (from [1])
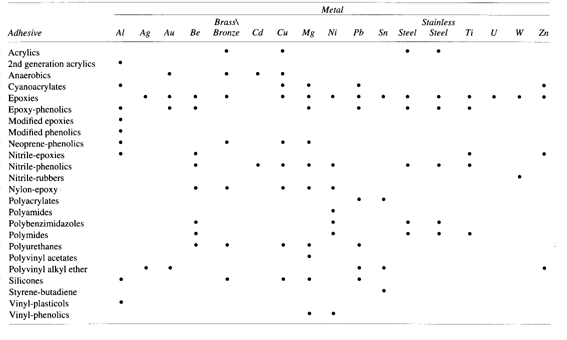
Figure 3.4 Metal-adhesive compatibility chart (from [1])
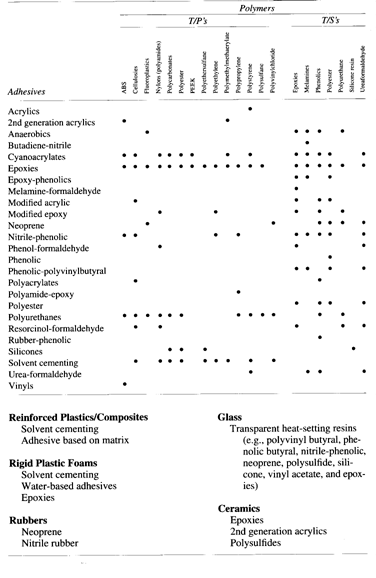
Figure 3.5 Non-metal/adhesive compatibility chart (from [1])
Notice from Figures 3.4 and 3.5 that the only adhesive judged to be compatible
with both stainless steel and ceramics is epoxy, currently used in the
piezoelectric motor. Some variations on the basic epoxy adhesive system exist.
Epoxy-polysulfone and epoxy-phenolic, both thermosetting alloys, are other
possible candidates for the piezoelectric motor system. These two alloys have
superior bonding characteristics with ceramics.
4. References
Robert W. Messler, Jr., Joining of Advanced Materials, (Stoneham,
Massachusetts: Butterworth-Heinemann, 1993) 553.