copyright 2003
Gary L. Bertrand
University of Missouri-Rolla
copyright 2003
Gary L. Bertrand
University of Missouri-Rolla

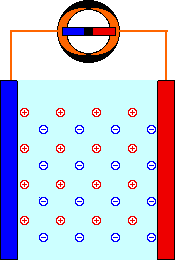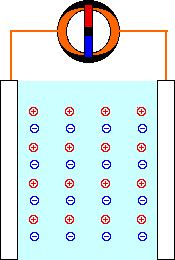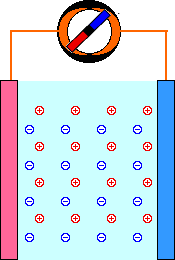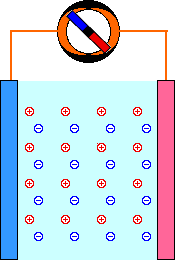
When a mild electric field (Volts, E) is applied across a pair of electrodes immersed in a solution, there is a tendency for current (Amperes, I) to flow. This current is carried through the metal wires by electrons, but electrons are not able to move through the solution. The current through the solution must be carried by ions. Part of the current is carried by negatively-charged anions moving in the same direction as the electrons. The rest of the current is carried by positively-charged cations moving in the opposite direction.
The amount of current that is observed depends on a number of factors:
1. The current (I) is directly proportional to the voltage (E).
2. The current (I) is directly proportional to the area of the electrodes (A).
3. The current (I) is inversely proportional to the distance between the electrodes (d).
4. The current (I) is roughly proportional to the concentrations of the ions (C).
This suggests an equation relating these terms:
I = constant xExAxC/d ,
in which the constant depends
on the solvent,
the nature of the ions, and
the temperature.
The ratio of voltage to current is defined as the electrical resistance (E/I = R), so the ratio of current to voltage is the reciprocal of the resistance:
I/E = 1/R = constant x C x (A/d) .
The quantity A/d is determined by the construction of the electrodes, and is called the cell constant (Kcell, cm) with dimensions of cm2/cm.
Conductivity is defined as 1/R. It has the units of ohms-1, and was originally called mhos, but is now defined as Siemens (S).
The specific conductivity (K,
kappa)
is defined as the conductivity between electrodes of 1 cm2 area
and 1 cm apart:
K = d/AR
= 1/(R x
Kcell)
,
with dimension S/cm , and
K = constant x C .
When the concentration of the ions is given in moles/cm3, the constant is called the molar conductivity and has the dimension of S-cm2/mole.
It is more common to use a term called the equivalent conductivity (L , lambda , S-cm2/equivalent)
K = L x C x N ,
in which N is a simple factor for converting from moles to equivalents:
N = 1 for 1:1 electrolytes such as HCl, KBr, NaNO3, etc.
N = 2 for H2SO4, BaCl2, PbSO4
N = 3 for Al(OH)3, H3PO4
N = 6 for Al2(SO4)3
Precise measurements show that the equivalent conductivity varies slightly with concentration, approaching a limiting value at infinite dilution (Lo), and increases with increasing temperature. The concentration of ions is equal to the equivalent concentration for an ionic compounds which ionizes completely (strong electrolyte), but for a partially-ionized compound (weak electrolyte) the concentration of ions may be much smaller than the equivalent concentration of the compound.
The value of L provides a measure of how easily the ions move through the solution. A simpler comparison can be made by measuring the conductivities of solutions of different ionic compounds at the same equivalent concentration. This comparison can be made at any concentration, but with most commercial instruments with a cell constant of unity (Kcell = 1) a convenient concentration is 0.001 moles/Liter for 1:1 electrolytes and 0.0005 moles/Liter for divalent electrolytes (sulfates and barium or calcium salts).