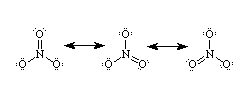
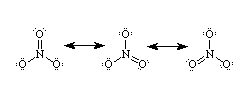
I can't make the web insert extra spaces so I'll use ampersands.
&&&&&&..-&&&&&&&&&&&&&&&&..-&&&&&&&&&&&&&&&&&&&&&&&&&&
&&&&..O..&&&&&&&&&&&&&&..O..&&&&&&&&&&&&&&&..O..&&&&&&
&..&&&|&&&..-&&&&&&-..&&&|&&&..&&&&&&&&-..&&&||&&..-&&
&&O&=&N&-&O..&&&&&&..O&-&N&=&O&&&&&&&&&..O&-&N&-&O&..&
&..&&&+&&..&&&&&&&&&..&&&+&&&..&&&&&&&&&..&&&+&&&&..&&
What is so important to note that the positive charge goes on the the very electronegative N (usually nitrogens don't have to put up with accepting positive charges) because oxygen is even more electronegative.