The bond angle of a tetrahedron is
______ degrees.
The best arrangement of a given number of electron pairs is the one that
minimizes/maximizes the repulsions among
them.
T or F The molecular geometry is the
arrangement of electron pairs around the central atom.
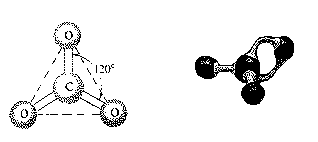
This structure can be described as trigonal planar,
tetrahedral, or trigonal pyramidal.
T or F A double or triple bond is counted as
one bonding pair when prediciting the geometry.
An example of a linear model would look like this: 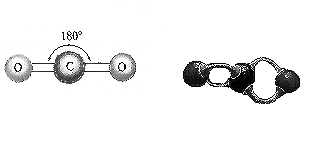
and could possible be increases/decreases as the
number of nonbonding electron pairs increases.
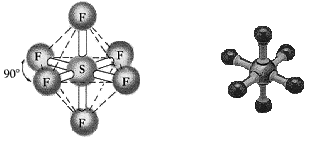
This structure contains more than four electron pairs around it and can be
described as
equitorial/axial positions.
A molecule has one end with a slight positive charge and one end that is
slightly negative. This molecule can be classified as T or F Polar molecules align themselves in an
electric field with their negative ends pointing toward the positive plate.
What does the _________?
What are the two types of bonds?
___________.
The bonding sigma molecular orbital is higher/lower in energy than the atomic one-s orbital.
Half of the differece between the number of bonding and antibonding electrons is
known as _________
whereas 



