H H O H H | | [O] / \ H+ | | C = C -------> - C - C - -----> - C - C - | | silver water | | H H catalyst OH OHNote that ethylene glycol is very toxic, and that household pets and possible small children are attracted to the pretty green color, and the sweet pleasant taste, leading to fatality.
H H O CH3 | | [O] / \ | C = C + -----> C - C - C + H O ----> HO - C - CH - OH | | catalyst 2 | 2 H CH3 HPropylene glycol is less toxic.
H H H H
| | | |
HO - C - C - O - C - C - OH
| | | |
H H H H
Neopentylene glycol (2,2- dimethyl propane 1,3-diol) improves resistance
to thermal degradation.
H CH3 H
| | |
HO - C - C - C - OH
| | |
H CH3 H
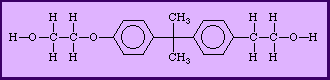
Another diol, 2,2- bis (4-hydroxyphenyl) propane, is shown below:
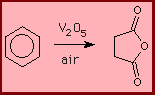
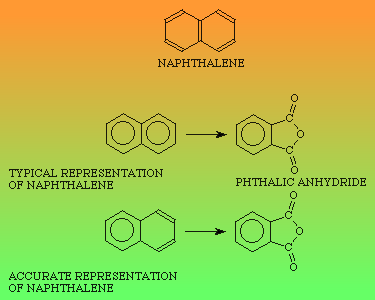
H O H O OH H OH
| / \ 2 | | | the product is isolated by
H - C - C - C - H -----> H - C - C - C - H distillation under reduced
| | | | | | pressure.
H H H H H H
Propylene glycol is the preferred diol because it forms polyesters which
are compatible with styrene and which show little tendency to crystallize,
and also, it is readily available at low cost.
propylene glycol 100 parts maleic anhydride 72 parts phthalic anhydride 54 parts
O O
// ||
R1 - C R1 - C - O - C - C - OH
\
|| O + HO - C - C - OH ----> ||
/
R2 - C R2 - C - OH
\\ ||
O O
O
||
R1 - C - O - C - C - OH
----> ||
R2 - C - O - C - C - OH
||
O
+2 * +3 Co + HOOH ----> R-O + OH- + Co O O || +3 || * +2 + R - OH + Co ----> R - O + Co + HEither one of the above radical species can go on and initiate the crosslinking reaction. The cycle is repeated until all the peroxide is decomposed.
++ +++ -
H O + Fe --> Fe + OH
2 2
++ +++ -- -
S O + Fe --> Fe + SO + SO .
2 8 4 4
Unsaturated polyesters
O O O O
|| || || ||
HO - C - R - OH + HO - R - C - OH ---> HO - C - R - C - OH + HO - R - OH
the diol distills from the mixture as the diacid is formed.
O H H
/ \ | |
H - C - C - H + H O ---> H - C - C - H
| | 2 | |
H H OH OH
ethylene oxide ethylene glycol
Synthesis of dimethyl terephthalate- xylene feedstock
CH
| 3
OH O
| |
CH C=O C=O
| 3 | |
benzene ----> benzene ----> benzene
| | |
CH C=O C=O
3 | |
OH O
|
CH
3
xylene dimethyl terephthalate
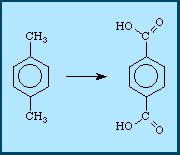
In an alternate process, the starting material is once again, p-xylene,
but there are four steps instead of two:
O O || || - C - O - R1 + R2 - OH ---> - C - O - R2 + R1 - OHSynthesis of Poly(ethylene terephthalate) (PET)- from ethylene glycol and terephthalic acid feedstocks
H H O O H H O O
| | || || | | || ||
2 H - C - C - H + HO - C - benzene - C - OH ---> -[- O - C - C - O - C - benzene - C -]- + water
| | | |
OH OH OH OH
In an alternate scheme, the diacid above is replaced with the corresponding
dimethyl ester:
O O
|| ||
- C - OH becomes - C - O - CH
3
and methanol is given off instead of water.A A A A A A A A B B B B B B B B (first round of reaction) A-B A-B A-B A-B A-B A-B A-B A-B (second round of reaction) A-B-A-B A-B-A-B A-B-A-B A-B-A-B (third round of reaction) A-B-A-B-A-B-A-B A-B-A-B-A-B-A-B (fourth round of reaction) A-B-A-B-A-B-A-B-A-B-A-B-A-B-A-B
A A A A A A A B B B B B B B B B (first round of reaction) A-B A-B A-B A-B A-B A-B A-B B B (second round of reaction) B-A-B A-B-A-B A-B-A-B A-B-A-B B (third round of reaction) B-A-B A-B-A-B-A-B-A-B B-A-B-A-B (fourth round of reaction) B-A-B-A-B-A-B-A-B-A-B B-A-B-A-B
A A A A A A A B B B B B B B B B (first round of reaction) A-B A-B A-B A-B A-B A-B A-B B B (second round of reaction) B-A-B A-B-A-B A-B-A-B B-A-B A-B (third round of reaction) B-A-B-A-B-A-B A-B-A-B B-A-B-A-B (fourth round of reaction) B-A-B-A-B-A-B-A-B-A-B B-A-B-A-BAnd now, we prevent the excess B from reacting until the end
A A A A A A A B B B B B B B B B (first round of reaction) A-B A-B A-B A-B A-B A-B A-B B B (second round of reaction) A-B-A-B A-B-A-B A-B-A-B A-B B B (third round of reaction) A-B-A-B-A-B-A-B A-B-A-B-A-B B B (fourth round of reaction) A-B-A-B-A-B-A-B-A-B-A-B-A-B B B (fifth round of reaction) B-A-B-A-B-A-B-A-B-A-B-A-B-A-B B