| white | red | yellow | green | |||||
| Titanium Dioxide Calcium Carbonate Lead Sulfate | Iron Oxide (Fe[x]O[y]) | Lead Chromate | Cobalt salts Chromium salts | |||||
OH
H H H H | H H hydrogen OH OH OH
| | O2 | | C-C-C | | peroxide | | |
C = C ------> C = C ------> C = C --------> C - C - C
| | catalyst | | | | 60-70 C
H CH3 H C=O H CH2 glycerol
| | isolated by
H OH distillation
propylene acrolein
Glycerol has a boiling point of 290 deg C, and is a colorless liquid that
can explode on contact with strong oxidizing agents such as chromium
trioxide, potassium chlorate, or potassium permanganate.
OH OH
| |
CH2 H - C - H
| |
HO - CH2 - C - CH2 - OH H - C - OH
| |
CH2 HO - C - H
| |
OH H - C - OH
|
H - C - OH
|
H - C - H
|
OH
Pentaerythritol Sorbitol
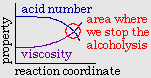
The previously seen reaction which
converts benzene to maleic anhydride also converts naphthalene to
phthalic anhydride at high temperatures.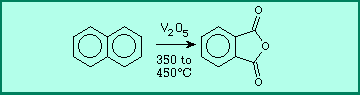
H O
| ||
H - C - O - C - R C - OH
| |
| O |
| || excess base |
H - C - O - C - R --------> C - OH
| water |
| O |
| || |
H - C - O - C - R C - OH
|
H glycerol
Natural oils
Oils which find commercial utilization are of animal and vegetable origin
H O
| ||
H - C - O - C - R C - OH
| |
| O | O
| || 3 NaOH | || - +
H - C - O - C - R --------> C - OH + 3 R - C - O Na
| water |
| O |
| || |
H - C - O - C - R C - OH
|
H glycerol
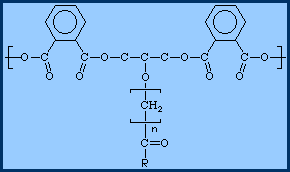
Types of alkyd resins| drying oil resins | mostly unsaturated |
| semi-drying oil | resins- mixture |
| non-drying oil resins | mostly saturated oil content |
| short oil | contain less than 50% oil content |
| medium oil | contain 50%- 70& oil content |
| long oil | contain greater than 70% oil content |
| drying oil resins | short oil resins | soluble only in aromatic solvents cured at elevated temperatures give very hard, glossy finishes used in finishes for appliances, signs and toys |
| drying oil resins | medium oil resins | soluble in aliphatic-aromatic blends may be air-dried or heated give durable, glossy finishes used on finishes for farm implement, hardware, and metal furniture |
| drying oil resins | long oil resins | soluble in aliphatic solvents (eg. naphtha) have good brushing characteristics dry rapidly in air give reasonably durable, glossy films used in household paints |
| semi-drying oil resins | no division based on oil length | give films with improved resistance to yellowing on aging used particularly for high gloss white finishes |
| non-drying oil resins | short oil resins | soluble only in aromatic solvents used mainly in conjunction with amino resins give improved adhesion and flexibility used in stoving finishes for appliances |
| non-drying oil resins | medium oil resins | soluble in aromatic solvents used mainly as plasticizers for cellulose nitrate for furniture finishes |
| non-drying oil resins | long oil resins | ----- does not specify if
|
H O
| ||
H - C - O - C - R C - OH
| |
| O | O
| || | ||
H - C - O - C - R --------> C - OH + 3 HO - C - R
| |
| O |
| || |
H - C - O - C - R C - OH
|
H glycerol