 A report on polyurethane by Darren Schilberg of Penn State
, provides a lot
of interesting information.
A report on polyurethane by Darren Schilberg of Penn State
, provides a lot
of interesting information.
: :
O
: || :
- N - C - O -
| :
H
urethane
Polyurethanes can be classified into the following major groups:
O O
|| ||
O=C=N - R - N=C=O + HO - R - OH --> -[- C - N - R - N - C - O - R - O -]-
|
H
diisocyanate diol polyurethane
Isocyanates will react with an amine to form a polyurea:
H H O O
| | || ||
O=C=N - R - N=C=O + H-N - R - N-H --> -[- C - N - R - N - C - N - R - N -]-
| | | |
H H H H
diisocyanate diamine polyurea
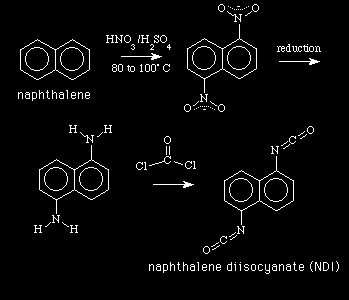
Synthesis of isocyanates
O O
|| ||
R - N - H + Cl - C - Cl ---> R - N - C - Cl + HCl
| |
H H
amine phosgene isocyanate hydrochloric
precursor acid
O
||
R - N - C - Cl ---> R - N = C = O + HCl
|
H
isocyanate isocyanate
precursor

O
H || H
| Cl - C - Cl |
H N -[- C -]- NH + ----------> O=C=N -[- C -]- N=C=O
2 | 6 2 |
H H
hexamethylenediamine hexamethylene diisocyanate
HDI sees limited use in the preparation of moulding materials.
O
C - OH / \ C - O -[- C - C - O -]-m H
C - C
| epichlorohydrin | n,m range from 1 to 10
------------>
C - OH C - O -[- C - C - O -]-n H
diol low molecular weight polyether
The same trick can be done with glycerol (1,2,3-trihydroxypropane), which
would have three polyether chains off the glycerol unit. The molecular
weight of this is 1000 to 3000.
O O O O
|| || || -CO2 ||
R - N = C = O + R - C - O - H ----> R - N - C - O - C - R ----> R - N - C - R
| |
H H
isocyanate carboxylic acid unstable product amide
An amide can react with isocyanate, as seen further on.
O
||
R - N = C = O + H - O - H ----> R - N - C - O - H ----> R - N - H
| |
H H
isocyanate water unstable product amine
An amine can react with isocyanate, as seen further on.
O O O
|| || ||
R - N = C = O + R - N - C - O - R -----> R - N - C - N - C - O - R
| | |
H H H
isocyanate urethane allophanate
O
||
R - N = C = O + R - N - C - N - R ---> What is drawn in the notes does
| | not make sense. The word "biuret"
H H is used.
O O O
|| || ||
R - N = C = O + R - N - C - R ----> R - N - C - N - C - R
| | |
H H R
O
||
R - N = C = O + HO - benzene ----> H - N - C - O - benzene
|
R
Dimerization of isocyanate
O
||
C
/ \
2 R - N = C = O ---> R - N N - R
\ /
C
||
O
uretidione
Trimerization of isocyanate
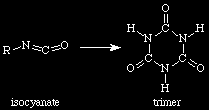
C-C / \ N-C-C-N \ / C-CBoth polyesters and polyethers can be used. However, polyethers are hydrolytically susceptible.??
O
/ \
-- C - C
epoxy
Applications of Epoxides include adhesives, paints, coatings,
plastics, and extremely strong glues used for woodwork.
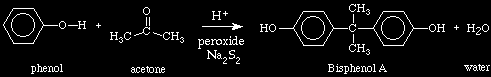
base
C = C - C + Cl ---> C = C - C - Cl + HCl ---->
2 HOCl
O
/ \
----> C - C - C - Cl
epichlorohydrin (reacts with bugs and humans)
Resin Preparation: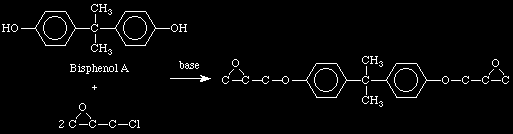
CH3
+ - | - +
Na O - benzene - C - benzene - O Na
|
CH3
And as you would deduce, the reaction of two epichlorohydrins with
the above would produce a NaCl byproduct.
O / \ C - C.We've discussed exact stoichiometric match and step polymerization before. It should make sense that if the Bisphenol A to epichlorohydrin to base stoichiometry is exactly 1:1:1, then the whole system would form one molecule.
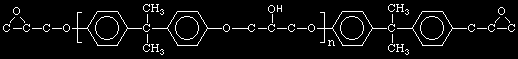 Notice that the variable 'n' corresponds to the number of OH groups
on the DGEBA molecule.
Notice that the variable 'n' corresponds to the number of OH groups
on the DGEBA molecule.
| Molar ratio- epichlorohydrin/Bisphenol A | Molecular Weight | Softening Point |
| 10 : 1 | 370 | 9 deg C |
| 2 : 1 | 451 | 43 deg C |
| 1.4 : 1 | 791 | 84 deg C |
| 1.33 : 1 | 802 | 90 deg C |
| 1.25 : 1 | 1133 | 100 deg C |
| 1.2 : 1 | 1420 | 112 deg C |